《Annals of Psychiatry and Mental Health》於2020年12月發表了一項關於CES治療失眠的新薈萃分析。該研究包括隨機對照試驗(RCT)以及非隨機干預研究(NRSI)。
A Meta-Analyses of Cranial Electrotherapy Stimulation in the Treatment of Insomnia
Price LR, Briley J, Hitching R. A Meta-Analyses of Cranial Electrotherapy Stimulation in the Treatment of Insomnia. Ann Psychiatry Mental Health. 2020; 8(3): 1157. Download Article
Funding Source, Location of Study or Author’s Affiliation
Electromedical Products International, Mineral Wells, Texas
Device
Alpha-Stim®
Key Variable
Insomnia
Objective
Summarize the scientific data on Alpha-Stim Cranial Electrical Stimulation (CES) as a treatment of insomnia by performing a systematic review of the literature. Determine the efficacy of Alpha-Stim CES by conducting meta-analyses of the available studies using Alpha-Stim as a treatment for insomnia.
Design
A systematic literature review including Randomized Controlled Trials (RCTs) and Non-Randomized Studies on Interventions (NRSIs) for the use, effectiveness, and the risk/benefit of Alpha-Stim CES in the treatment of insomnia disorders. Following Cooper’s Taxonomy of Literature Reviews, appropriate for the behavioral and physical sciences and the PRISMA reporting guidelines. As CES devices differ significantly in their electrical outputs and usage, individual assessment is warranted. Accordingly, the meta-analysis was limited to one CES device for the treatment of insomnia.
Primary Outcome Measure
Treatment of insomnia using Alpha-Stim CES, including
- Level of use
- Effectiveness
- Risk/benefit
Secondary Outcome Measure
None reported.
Key Inclusion Criteria
To be included in this meta-analysis, studies were:
- RCTs – inclusive of subjects blinding (with a description of how blinding was implemented), a sham versus active condition
- Use of valid and reliable measurement instruments
- NRSIs with patients exhibiting symptoms of insomnia.
- A pretest-posttest design (additional repeated measures were acceptable)
- Rated as “good” or “fair.”
Key Exclusion Criteria
None reported
Protocol Summary
- A computer-based search of MEDLINE and EMBASE databases since their beginning.
- A search of the Cochrane Central Register of Controlled Trials (CENTRAL) included in the Cochrane Library.
- The search proceeded within abstract, subject terms, and titles of studies and reports published in peer-reviewed journals between January 1, 1981, and August 26, 2020. Keywords: Insomnia and Alpha-Stim and cranial electrotherapy stimulation and randomized control trial or nonrandomized or open-label or case study.
- Screening references are given in relevant systematic reviews and identified RCTs.
- Citation tracking of identified RCTs using the Science Citation Index through the Web of Science.
- A scoring rubric for each study was applied using the guidelines from Zaza et al., (2000) with scoring categories of 0-1 limitations (rating = good); 2-4 limitations (rating = fair); 5-9 limitations (rating = limited) in the selection of the research studies to be included in the meta-analysis. The Revised Cochrane Risk-of-Bias Tool was used to inform the decision to include a study within an RCT design.
Device Application Protocol
The evaluation of strengths and limitations of the research studies included in the meta-analyses adhered to guidelines published by Zaza et al., (2000), the Cochrane Handbook for Systematic Reviews of Interventions, and in the Handbook of Research Synthesis and Meta-Analysis.
Statistical Analysis Plan
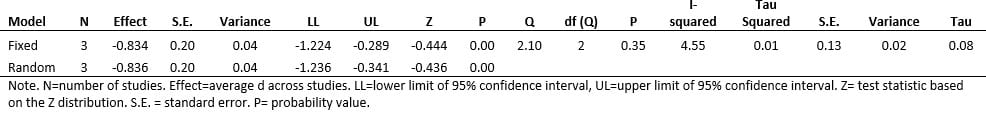
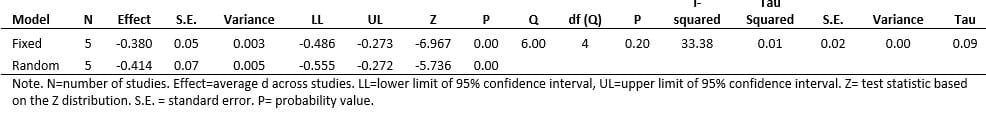
A complementary approach to synthesize the meta-analytic results from the NRSIs with RCTs was used. Cohen’s d effect size summary metric was used in all analyses. Fixed and random effects models, inclusive of homogeneity of Cohen’s d effect sizes are reported on 8 studies found in the CENTRAL registry using Cooper’s Taxonomy of Literature Reviews. The meta-analyses were performed using the Comprehensive Meta-Analysis, version 3 program.
Results
Subjects
The inclusion criteria yielding 3 RCTs and 5 NRSIs. The RCTs had a total sample of N=163, and the 5 NRSIs had a total of 376 participants.
Data Analysis
The studies used in the meta-analyses all had significant outcomes of p<0.05 through p<0.001 for insomnia and many also revealed equally good effects for the treatment of anxiety and depression.
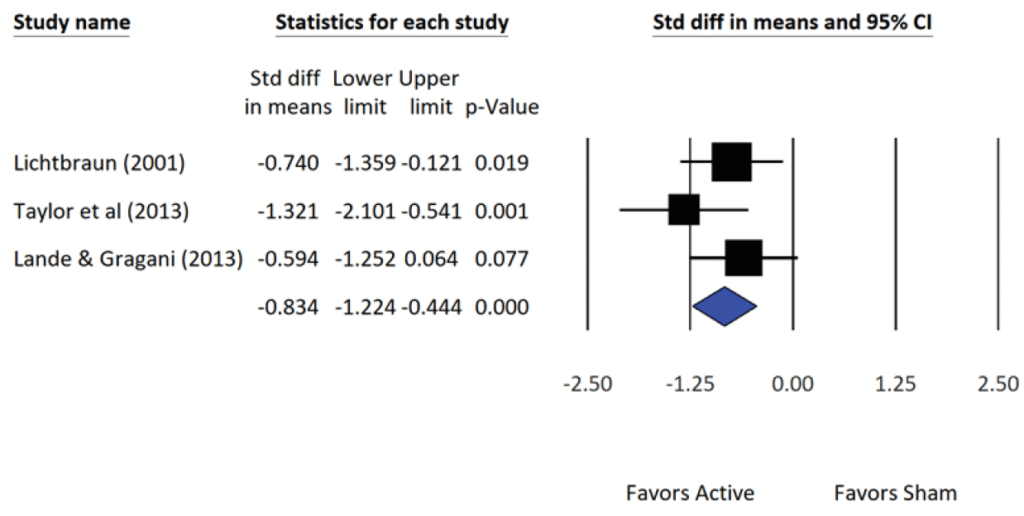
The meta-analytic results of the three (N=3) RCT studies on insomnia and the accompanying statistical summary show a large effect in favor of the active treatment group. The average (population) effect for the 3 studies was observed as d = -0.83 (i.e., the mean insomnia level at posttest for the active group was -0.83 standard deviations lower than the mean insomnia level of quality sleep for the sham group).
An effect size of -0.83 is classified as large. Given the congruency (i.e., closeness), between the summary statistics of Fixed- and Random-effects models. It is reasonable to also state that CES shows a large effect in favor of the active treatment group relative to reductions in insomnia.
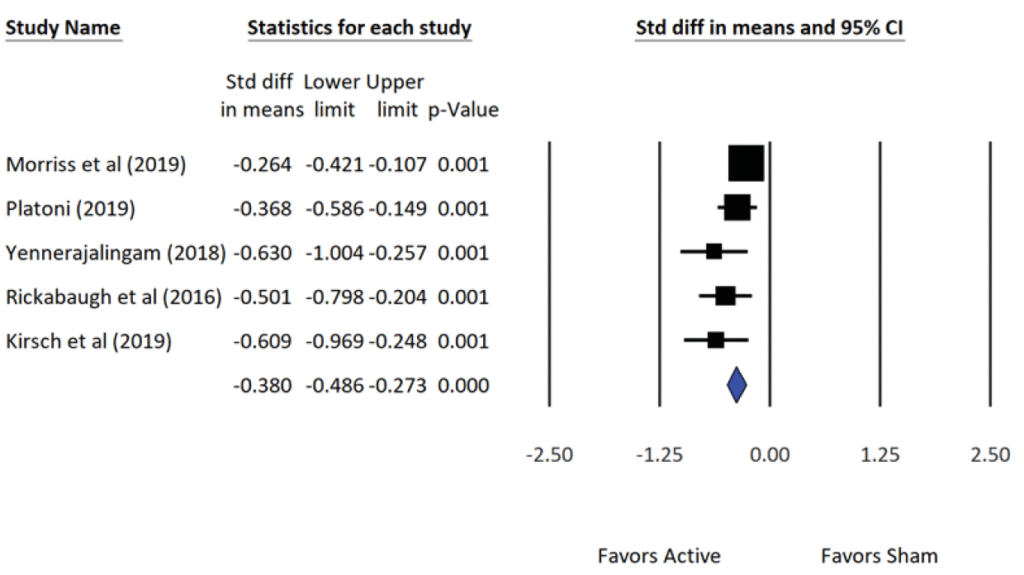
The five (N=5) NRSI studies included in this meta-analysis on insomnia and the accompanying statistical summary of the studies show a small effect in favor of the active treatment group. The average (population) effect for the Fixed-effects model was -0.38 (small), and for the Random-effects model, the average effect was -0.41 (medium).
Meta-Analyses Summary Statistics – Insomnia RCTs. Point estimate = average standard effect, d, over 3 studies. Q-value = test of study heterogeneity (i.e., are the set of effect sizes homogeneous). I-squared = magnitude of study heterogeneity (~25% = small; ~50% = medium; ~75% = large).
Meta-Analysis Summary Statistics – Insomnia NRSI. Point estimate = average standard effect, d, over 5 studies. Q-value = test of study heterogeneity (i.e., are the set of effect sizes homogeneous). I-squared = magnitude of study heterogeneity (~25% = small; ~50% = medium; ~75% = large).
The forest plots show for the RCTs and NRSIs show (a) the effect size d, (b) the variability of each study’s effect via the 95% confidence interval, and (c) the average (i.e., population estimate) effect size for all the studies (blue diamond). The symbols in the forest plot depict the relative weight for each of the studies (e.g., the larger the symbol the greater the relative contribution each study makes to the final result). Also informative is the width of the confidence interval. For example, the larger the sample size of an individual study, the smaller the width of the interval and the greater the precision of the effect size.
Forest Plot: Summary Statistics of Effect Sizes from Alpha-Stim CES RCTs of Insomnia (N=3).
Forest Plot: Summary Statistics of Effect Sizes from Alpha-Stim CES NRSIs of Insomnia (N=5).
The studies included in the meta-analyses report that patients receiving CES treatment have shown improvements in negative domains that typically cooccur with insomnia such as somatization, interpersonal sensitivity, obsessive or compulsive thoughts, excessive worry, hostility, fearfulness, alcohol and substance use, and paranoia. Concurrently patients report improvement in symptoms associated with CES treatment as measured by the Global Assessment of Function (GAF), a measure incorporated in some of the studies in the meta-analyses and by clinicians using the Clinical Global Impression (CGI), scale to report on patient improvement.
Conclusion
These meta-analyses of CES as a treatment for insomnia showing demonstrate a large (d = -0.83), average effect size for the 3 RCTs, in addition to a small (d = -0.38), average effect size for 5 NRSI studies in favor of the active CES treatment group. The effect sizes and Cohen’s d values were medium for the RCTs and small for the NRSIs. In comparison, the effect sizes typically associated with antidepressant medication for published studies is 0.37 (95% CI, 0.33 to 0.41), and for unpublished studies, it’s less than 0.15 (95% CI, 0.08 to 0.22), both qualifying as small. When the side effect profile of medications vs CES is considered, the supremacy of CES over medications is even more notable.
The risk profile for CES was virtually negligible, with mild and self-limiting vertigo or cervicogenic headaches when the current is too high, and skin irritation at the electrode site reported in less than 1% of patients.
The authors concluded that CES has a significant effect in the treatment of moderate to severe insomnia across a variety of patient populations and was significantly more effective than wait-list controls. In addition, CES is an effective treatment for insomnia in a range of populations with a spectrum of insomnia severity, as evidenced by pre-and post-scores of the appropriate insomnia measures based on the sample population – civilian, military, and first responders. Moreover, it is a useful adjunctive to other ongoing treatments including pharmacotherapy and psychotherapy for insomnia.
Limitations
Participants were self-selected resulting in possible selection bias. The RCTs had a limited number of patients with a significant diagnosis of disordered sleep. The NRSIs lacked randomization and a control group and many of the patients were continuing to receive other treatments (e.g., pharmacotherapy).
Study Quality: Good